Endotoxin Testing – Pyrogen Detection for Biological Safety
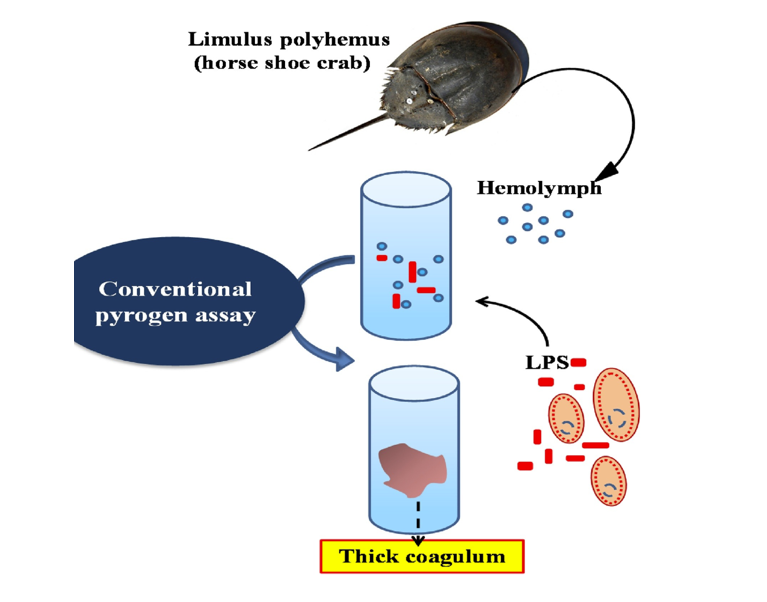
Even in the absence of live microbes, bacterial endotoxins—primarily lipopolysaccharides (LPS)—can introduce serious confounding variables into biological assays and animal studies. To address this, Pinnacle Compounds employs the Limulus Amebocyte Lysate (LAL) test, a highly sensitive assay derived from horseshoe crab blood that detects even trace quantities of endotoxins.
Our multi-layered approach to ensuring research-grade excellence.

Mass Spectrometry (MS) verifies the molecular weight and structure of each compound. This ensures the product exactly matches its intended identity.
Using ICP-MS, we screen for heavy metals like lead and mercury. This ensures our compounds are free from toxic elements and meet safety standards.
This technique is particularly essential when dealing with structurally similar analogues, where minor sequence variations or stereochemical differences can alter pharmacological behavior.

To safeguard research integrity, particularly in applications involving injection, cell culture, or sensitive in vivo models, all applicable products undergo stringent sterility testing
Each finished unit undergoes dual-layer visual inspection prior to packaging. This includes human review under high-lux lighting and magnification

This evaluation informs users of the most appropriate vehicle for their specific applications and is particularly critical for peptides that exhibit sequence-dependent solubility properties